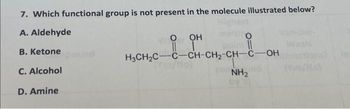
Understanding functional groups is pivotal in the study of organic chemistry, as they play an essential role in the behavior and properties of organic molecules. To determine which functional group is absent in a given molecular structure, one must engage in a meticulous analysis of the chemical composition and architectural configuration of that molecule. This exploration not only enriches your comprehension of organic compounds but also fosters a more nuanced understanding of biochemical reactions and mechanisms.
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. Each functional group imparts different properties to the compounds they are in, thus influencing their reactivity, polarity, and solubility. By identifying the functional groups present in a molecule, chemists can infer much about its potential reactions and interactions. However, this task can often lead to the enigmatic question: which functional group is conspicuously absent?
Before delving into this inquiry, it is essential to categorize major functional groups that commonly appear in organic chemistry. The most notable groups include:
- Hydroxyl group (-OH) – Found in alcohols, responsible for polar characteristics.
- Amino group (-NH2) – Present in amines and amino acids, playing a critical role in biological processes.
- Carboxyl group (-COOH) – Characteristic of carboxylic acids, contributing acidic properties.
- Ester group (-COO-) – Key in the structure of fats and oils, linking organic acids to alcohols.
- Carbonyl group (C=O) – Divided into aldehydes and ketones, influencing flavor and fragrance profiles.
- Alkene group (C=C) – Vital in producing unsaturated compounds, impacting stability and reactivity.
- Alkyne group (C≡C) – Found in compounds with triple bonds, known for their unique chemical properties.
Each of these functional groups can play an integral role in the overall behavior of an organic molecule. When presented with a molecular structure, one needs to scrutinize the components meticulously to distinguish which of these building blocks are present and to ascertain the absence of others. A fundamental approach to this analysis begins with identifying the core framework of the molecule, often indicated by carbon atoms and their bonding.
To illustrate this method, consider a hypothetical molecule that seemingly exhibits characteristics of a common organic compound. If the molecular structure showcases a predominance of carbon and hydrogen atoms, and you identify functional groups such as hydroxyl and carboxyl, you may wonder if any significant group is missing. For instance, if the structure lacks an amino or carbonyl group, it raises questions about the molecule’s potential interactions and functionalities. Knowing that such groups contribute to hydrophilicity or catalytic activity can guide one in predicting reactivity.
In the endeavor to identify absent functional groups, one may employ various analytical techniques, including spectroscopy, chromatography, or molecular modeling. Each method enables a deeper understanding of the molecular architecture and promotes insight into the groups that may inherently alter the molecule’s behavior. For instance, infrared spectroscopy may reveal the presence of specific bonds indicative of certain functional groups while simultaneously suggesting the absence of others.
The implications of identifying absent functional groups extend beyond academic inquiry. In the realm of drug design, understanding the precise nature of molecular interactions is crucial. Elements missing from the structure might indicate a lack of biological activity or certain safety profiles in pharmacology. Hence, the absence of a functional group can bear significant weight in the development of therapeutics.
Furthermore, the absence of functional groups can often lead to unexpected results during chemical reactions. A molecule lacking a reactive functional group may undergo alternative pathways, resulting in products that differ widely from anticipated outcomes. This phenomenon highlights the necessity of identifying these functional groups, and their absence, not merely as an academic exercise but as a crucial aspect of practical organic chemistry.
Conclusively, the detection of absent functional groups within a molecule invites a profound investigation into the qualities, reactivities, and potential applications of that compound. Whether through meticulous visual examination or sophisticated analytical methods, the quest to ascertain which functional groups are present or absent allows chemists and researchers to unlock the endless possibilities inherent in organic compounds. By fostering a keen eye for detail and pursuing inquiries into molecular structures, one can transform passive observations into active explorations of chemical potential.
Thus, the upcoming challenge lies in the quest for understanding which particular functional group eludes the molecular portrait presented. It is this very curiosity that drives the field of chemistry forward, urging scientists to explore uncharted territories and push the boundaries of molecular science.
