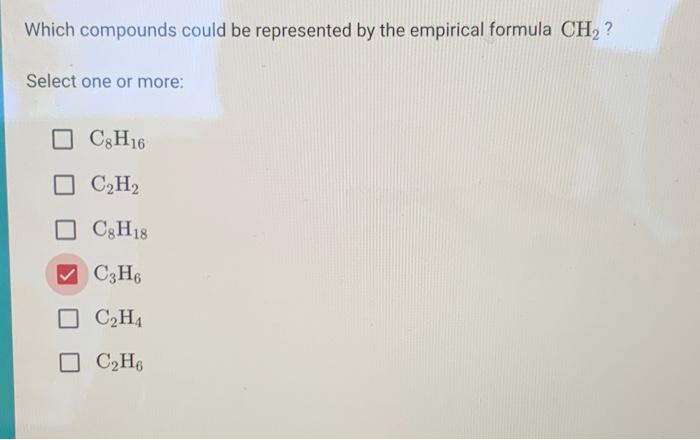
When we encounter the empirical formula CH₂, it evokes an intriguing question: Which compounds could this formula represent? The simplicity of two hydrogen atoms bonded to a single carbon atom belies the complexity and diversity of potential chemical species it encapsulates. To explore this topic, we must dive into the nuances of empirical formulas, molecular representations, and the interplay of chemical ratios that give rise to various compounds. This exploration not only sheds light on the formula CH₂ but also challenges our understanding of chemical bonding and molecular structure.
Empirical formulas provide a succinct method to convey the ratio of elements within a compound. Unlike molecular formulas, which indicate the actual number of each type of atom present in a molecule, empirical formulas offer merely the simplest whole-number ratio. In the case of CH₂, the ratio is one carbon atom to two hydrogen atoms. This seemingly straightforward combination unveils a plethora of possibilities when we consider various structural arrangements and functional groups.
To unpack the implications of the empirical formula CH₂, it is essential to contemplate the types of compounds characterized by this ratio. The most prominent class of compounds represented by CH₂ is alkenes, specifically those that contain a carbon-carbon double bond. Ethylene (C₂H₄) serves as the archetype for this category, displaying a vital role in both industrial applications and biological systems. Ethylene’s double bond allows for a unique reactivity profile, leading to various synthetic pathways and polymerization processes.
Moreover, CH₂ can also depict cycloalkanes such as cyclopropane (C₃H₆). Cyclopropane, a three-membered carbon ring, challenges traditional bonding theories due to angle strain inherent in its geometric configuration. This strain, coupled with characteristics like flammability and anesthetic properties, underscores the diversity of compounds that can stem from this empirical formula.
The realm of CH₂ extends beyond hydrocarbons to include functionalized compounds. A pivotal example is methanol (CH₃OH), a vital alcohol that showcases the influence of functional groups on molecular behavior. In this instance, the addition of a hydroxyl (-OH) group to the carbon atom transforms the simple hydrocarbon into a polar molecule with distinct physical and chemical properties. Methanol, being a protic solvent, plays an indispensable role in both industrial synthesis and biological processes, further emphasizing the impact of functionalization on compounds derived from CH₂.
Yet, the compounds represented by CH₂ do not merely reside in hydrocarbon chemistry. Consider, for instance, the effect of unsaturation. The presence of unsaturation—whether from double bonds, rings, or functional groups—affects the nature and reactivity of compounds derived from the empirical formula. This leads to a complex interplay between structure and reactivity, highlighting the importance of molecular geometry in predicting chemical behavior. Compounds such as acetylene (C₂H₂) illustrate this dynamic vividly, with its linear geometry resulting from a triple bond between two carbon atoms, despite originating from the same empirical ratio as CH₂.
As we advance our discussion, it is prudent to consider how the versatility of CH₂ extends to its representation of even more varied species through isomerism. Isomers—compounds with the same molecular formula but different structures—challenge the idea that a singular empirical formula delineates a single compound. For instance, C₄H₈ encompasses numerous structural isomers, including butene and cyclobutane, each associated with unique physical and chemical attributes stemming from their connectivity.
Moreover, the stereochemistry of isomers introduces another layer of complexity to CH₂ derivatives. Geometric isomerism, a phenomenon arising from restricted rotation around double bonds, presents fascinating challenges regarding compound reactivity and selectivity. The distinction between cis and trans forms can lead to drastically different properties and applications, accentuating the influence of spatial arrangement in chemical entities.
Furthermore, the synthesis of compounds with the empirical formula CH₂ can incorporate modern techniques and innovative pathways. The advent of catalytic processes and green chemistry has opened new avenues for establishing and manipulating carbon-hydrogen frameworks. As we harness these methods to cultivate sustainable practices within chemistry, the significance of CH₂ becomes ever more relevant in our endeavor to create novel compounds reliably and efficiently.
In an academic context, grappling with the ambiguity of CH₂ presents an intellectual challenge. It invites chemists to consider not just the molecules themselves, but also the broader implications of molecular design, synthesis, and application. The compounds represented by this empirical formula reflect a rich tapestry of scientific inquiry that transcends mere representation. It compels reflection on how we perceive chemical entities and the myriad pathways that link empirical ratios to functional compounds.
In conclusion, the empirical formula CH₂ embodies a fascinating intersection of simplicity and complexity within chemistry. Its implications stretch across a variety of functional classes and structural forms, prompting questions about molecular behavior, reactivity, and synthesis. As we unpack the potential representations of CH₂, we not only enhance our understanding of chemical ratios but also embrace the nuanced beauty of molecular interrelationships inherent in the world of chemistry. Compounds derived from this formula serve not only as a testament to the diversity of chemical structures but also as a catalyst for innovation and discovery in the scientific realm.
