Understanding the trend in electron affinity across the periodic table requires delving into several underlying principles, considerations, and nuances. Electron affinity, a measure of the energy change when an electron is added to a neutral atom, is pivotal in determining an element’s reactivity, bonding capabilities, and overall chemical behavior. This article seeks to illuminate the intricate patterns that govern electron affinity, revealing a sophisticated interplay between atomic structure and affinity values.
The concept of electron affinity can seem esoteric, yet it plays a fundamental role in the realm of chemistry. As one traverses from left to right across a period, and subsequently down a group, the patterns in electron affinity illustrate distinct trends influenced by atomic size, nuclear charge, and electron shielding effects. As we embark on this exploration, prepare to shift your perspective on the periodic table as a static entity, and instead view it as a dynamic framework that encapsulates the subtle energies at play.
1. The Periodic Table: A Contextual Introduction
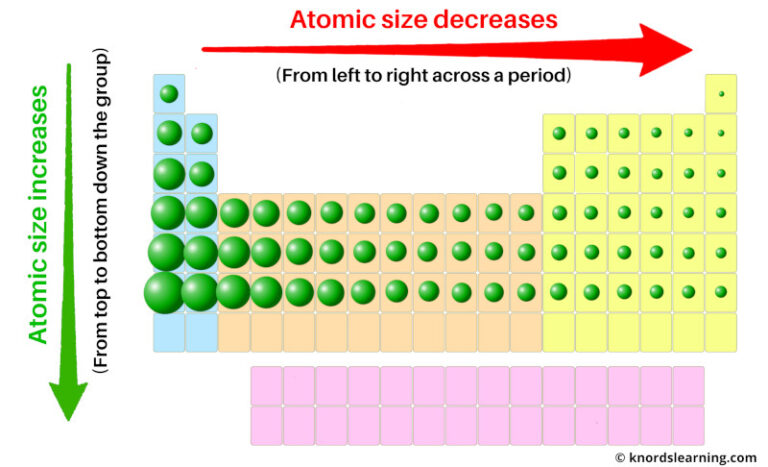
The periodic table is organized into periods (horizontal rows) and groups (vertical columns). Its structure reflects the electronic configuration of atoms, which in turn dictates their chemical properties, including electron affinity. Transitioning across a period, one observes an increase in nuclear charge as protons are added to the nucleus. Consequently, the pull exerted on the surrounding electrons becomes more substantial, generally resulting in a higher electron affinity.
2. Electron Affinity Trends Across Periods
In general terms, as one moves from left to right across a period, the trend in electron affinity is characterized by an increase in values. Elements on the left, such as alkali metals, possess low electron affinities. This is primarily due to their relatively large atomic radii and lower nuclear charge, which diminishes their ability to attract additional electrons effectively. In stark contrast, the halogens situated towards the right exhibit high electron affinities. This can be attributed to their elevated electronegativity and smaller atomic radii, which facilitate a stronger attraction for incoming electrons. The exception to this trend can be observed in certain noble gases, which, due to their filled valence shell, have negligible electron affinity.
3. Analyzing the Trend Down a Group
The vertical analysis of electron affinity reveals a different narrative. As one descends a group, the trend typically reflects a decrease in electron affinity values. This phenomenon can be ascribed to the increasing atomic radii and the consequential electron shielding, which lessens the effectiveness with which the nucleus can attract additional electrons. For instance, while fluorine has one of the highest electron affinities due to its modest atomic radius and significant electronegativity, its heavier counterparts like iodine exhibit notably lower electron affinities despite being in the same group.
4. The Impact of Atomic Size and Shielding Effects
Delving deeper, we can discern how atomic size and shielding significantly influence electron affinity. In larger atoms, the outer electrons are further removed from the nucleus and are thus subject to increased shielding by inner electrons. This shielding effect reduces the nucleus’s electrostatic pull on incoming electrons, leading to diminished electron affinities in lower periods. Consequently, understanding the interplay between these factors is crucial for a comprehensive grasp of electron affinity trends across the periodic table.
5. Exploring Anomalies in Electron Affinity Trends
It is essential to acknowledge that while the aforementioned trends provide a foundational understanding, several notable anomalies exist. For instance, the electron affinity values of nitrogen and phosphorus are surprisingly lower than one might anticipate based on their positions in the periodic table. This deviation can be attributed to the half-filled p-orbital stability in nitrogen, which diminishes its tendency to accept an extra electron. Such anomalies underline the importance of considering electronic configurations beyond mere periodic trends, offering a richer context for understanding chemical behavior.
6. Practical Applications and Relevance
The significance of electron affinity extends beyond theoretical implications; it plays a direct role in various chemical reactions and processes. In many cases, elements with high electron affinity are more likely to form anions. This tendency is pivotal in the synthesis of compounds. Understanding these trends can guide chemists in predicting elemental behavior, selecting appropriate reagents, and designing innovative materials. For instance, the enhanced electron affinity characteristic of halogens makes them ideal candidates for reactions involving electron transfer.
7. Concluding Thoughts: A Rich Tapestry of Interactions
In summation, the trends in electron affinity across the periodic table elucidate a complex tapestry woven from the fabric of atomic structure, electron interactions, and energy dynamics. As we’ve explored, these trends are not merely numerical values but reflections of deeper physical principles at play. By grasping the nuanced variations in electron affinity, one can acquire a more profound comprehension of elemental behavior and chemistry’s broader implications. Ultimately, this perspective shift empowers individuals in both academic pursuits and practical applications within the diverse field of chemistry, thereby piquing curiosity and fueling further exploration.
