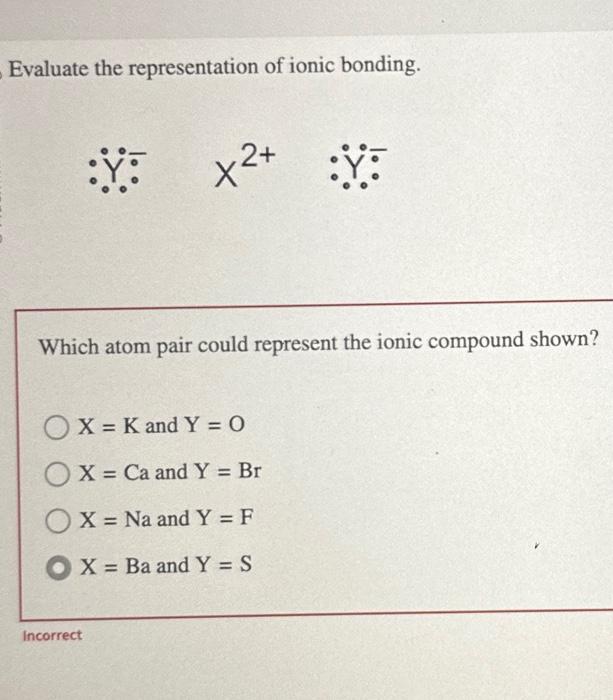
When pondering the nature of ionic bonding, one might ask: which atom pair could aptly represent the ionic compound being analyzed? This inquiry invites a delightful exploration of atomic interactions, elemental properties, and the fundamental aspects of chemical bonding. Central to this discussion is the understanding that ionic compounds form through the electrostatic attraction between positively charged cations and negatively charged anions. Such interactions lead us to a rich tapestry of possibilities, where elements from different groups of the periodic table showcase various bonding characteristics.
First, let us dissect the constituents of ionic bonds, beginning with the key players: metals and nonmetals. Typically, metals—located on the left side of the periodic table—tend to lose electrons and form cations. In stark contrast, nonmetals, found on the right side, usually gain electrons to become anions. This fundamental division provides the basis for a wide array of ionic compounds, each presenting unique properties and behaviors.
Equipment with this background knowledge, we can embark on our quest to identify suitable atomic pairs. Consider, for example, sodium (Na) and chlorine (Cl). Sodium, a highly reactive alkali metal, readily relinquishes one electron to achieve a stable electron configuration akin to that of neon. In doing so, it transforms into a sodium cation (Na+), characterized by a single positive charge. Conversely, chlorine, a halogen, possesses seven valence electrons and requires just one additional electron to attain a stable octet. Upon gaining this electron from sodium, chlorine becomes a chloride anion (Cl–). The resultant electrostatic attraction between Na+ and Cl– epitomizes the quintessential ionic bond, culminating in the formation of sodium chloride (NaCl), a widely recognized compound that exemplifies ionic character.
However, as we delve deeper, one might ponder whether this pairing is the only possible representation of ionic bonding. Is it plausible to envision other atom pairings yielding similar attractions? Naturally! Another prime candidate for ionic representation is magnesium (Mg) and oxygen (O). Magnesium, an alkaline earth metal, tends to lose two electrons to form a Mg2+ cation. On the flip side, oxygen, an essential nonmetal, with six valence electrons, seeks two additional electrons and thus adopts a charge of O2- upon completion of its octet rule. This results in a vigorous ionic bond between Mg2+ and O2-, ultimately giving rise to magnesium oxide (MgO), another solid illustration of ionic bonding dynamics in action.
Nevertheless, the complexity of ionic bonding extends beyond simplistic metallic and nonmetallic pairings. Contemplate alkaline metal compounds where lithium (Li) unites with phosphorus (P). While phosphorus is indeed a nonmetal, it possesses unique properties that allow it to engage in ionic bonding despite its tetravalent nature. Lithium, forming a Li+ cation, can bond with the phosphate ion (PO43-), an exceptionally stable polyatomic anion. Together, they produce lithium phosphate (Li3PO4), a representation of ionic structure even amidst notable complexity.
The consideration of ionic compounds propels us toward exploring the realm of electronegativity. The pursuit of identifying an atom pair for ionic compounds also necessitates acknowledging the disparities in electronegativity. As a playful challenge, consider this: is it possible to discover a pair with minimal electronegativity difference yet still form an ionic bond? Take the duo of aluminum (Al) and sulfur (S). Although aluminum’s electronegativity is relatively moderate compared to other metals, it still has the propensity to lose three electrons, while sulfur tends to gain two. In the case of Al and S, we observe the formation of Al2S3—another remarkable instance of ionic bond realization despite the less pronounced electronegativity gap.
As we journey through these elemental pairings, it remains critical to understand the overarching principles governing ionic bond formation. The aforementioned examples convey that the metalloid and nonmetal dynamic is not merely a formulaic approach but rather a nuanced interplay of atomic characteristics. Each pairing illustrates a variable range of ionic sizes, lattice structures, and reactivity patterns that contribute to the diverse properties of ionic compounds.
In conclusion, the query posed about which atom pair could represent the ionic compound leads us down an intriguing avenue of inquiry, showcasing a medley of suitable pairs such as Na and Cl, Mg and O, or even more intricate combinations like Li and P. By analyzing the underlying principles and electron configurations, one enhances both comprehension and appreciation for the realm of ionic bonding. The multifaceted nature of these interactions encourages curiosity and exploration, inviting both novices and seasoned chemists alike to delve deeper into the captivating world of chemical bonding. Armed with knowledge and insight, the challenge remains—what other atomic pairings hold the potential for ionic associations worthy of further exploration?
