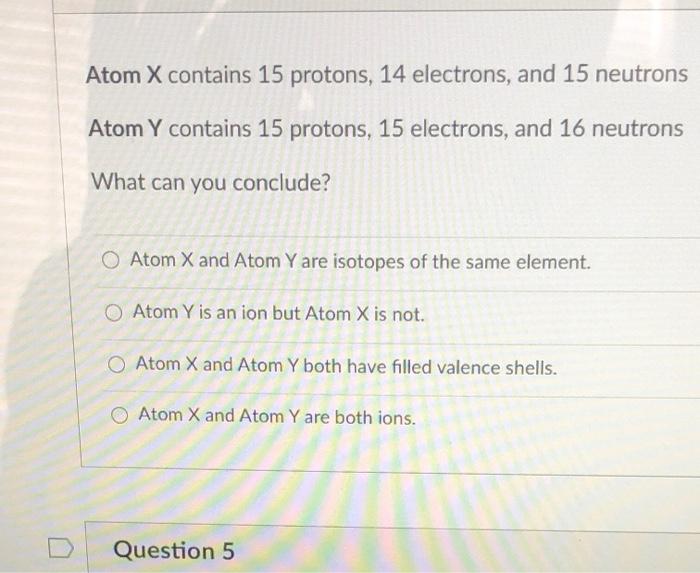
The study of atoms, the fundamental building blocks of matter, reveals a world of complexity and elegance. Each atom is uniquely defined by the number of protons it possesses within its nucleus. Among the various elements on the periodic table, one element that garners particular interest is phosphorus, characterized by its distinct identity of containing exactly 15 protons. Understanding phosphorus not only illuminates its role in various biological and chemical processes but also deepens one’s appreciation for the intricacies of atomic structure.
To grasp why phosphorus is so captivating, one must first understand the periodic table’s organization. Protons, along with neutrons and electrons, confer the elemental identity of an atom. The atomic number of an element corresponds exactly to the number of protons it contains, thus granting phosphorus its equivalent atomic number of 15. When delving into the properties and applications of phosphorus, one finds a compelling interplay between its chemical characteristics and its significance in both natural and manufactured phenomena.
Phosphorus exists in several allotropes, the two most recognized of which are white phosphorus and red phosphorus. White phosphorus, with its tetrahedral structure and highly reactive nature, is well-known for its property of igniting spontaneously upon exposure to oxygen. Conversely, red phosphorus is more stable and typically utilized in safety matches, fireworks, and various chemical syntheses. This dichotomy underscores the element’s fascinating capacity for transformation and the myriad ways it can be harnessed for diverse applications, from pyrotechnics to agriculture.
The role of phosphorus extends far beyond the laboratory and industrial applications. It is an essential element in biochemistry, playing a critical role in the formation of DNA and RNA, the molecules that constitute the genetic blueprint for all living organisms. Compounds of phosphorus, such as adenosine triphosphate (ATP), are integral to energy transfer within cells. This vital function imbues phosphorus with a profound importance in sustaining life, marking it not only as an element of interest but also as a cornerstone of biological existence.
The cultivation of phosphorus in agriculture is another fascinating aspect that highlights its significance. Phosphates, derived from phosphorus, are pivotal in fertilizers, promoting vigorous plant growth and supporting food production on a global scale. The application of phosphorus-rich fertilizers has revolutionized agricultural practices, allowing for increased yields and the support of the growing global population. However, this reliance also raises environmental considerations, as excessive use of phosphorus can lead to eutrophication in aquatic ecosystems, spurring unwanted algal blooms and degrading water quality. Thus, the story of phosphorus is entwined with both innovation and the impact on ecological balance.
Delving deeper, the exploration of phosphorus invites speculation about its cosmic implications. Phosphorus is one of the essential elements formed during stellar nucleosynthesis, illustrating its origins in the stars. As stars undergo their life cycles, they synthesize elements in their cores, distributing them across the cosmos through supernova explosions. Thus, the phosphorus in our DNA and the agricultural fertilizers we depend on can trace its lineage back to the very stars that illuminated the night sky. This cosmic connection elevates the discussion of phosphorus from mere chemical properties to a geophysical narrative, engaging our curiosity about the universe and our place within it.
Moreover, the discovery of phosphorus in various natural sources further enriches our understanding of this element. It is predominantly found in nature as phosphate rock, a vital mineral containing minimal but significant amounts of phosphorus. Mining and processing these rocks yield phosphates, which are crucial for industrial applications. Organizations and businesses continuously seek sustainable practices and efficient mining techniques to reduce the environmental impact while fulfilling the demand for phosphorus. This intersection of industry and ecology embodies the ongoing dilemma of resource management and sustainability.
As we navigate the complexities associated with phosphorus, it is essential to consider the implications of phosphorus pollution and resource depletion. The ‘Phosphorus Paradox’ suggests that while phosphorus is essential for life and agricultural productivity, excess phosphorus leads to environmental degradation, highlighting the necessity for responsible stewardship. Current research in sustainable agricultural practices aims to mitigate the adverse impacts while enhancing phosphorus recovery and recycling, fostering a holistic approach to elemental management and ecological preservation.
In conclusion, the exploration of phosphorus, the atom with 15 protons, serves as a gateway to understanding the fundamental principles of chemistry, biology, and environmental science. This small yet mighty element embodies a range of applications and implications, from its role in ecosystems to its cosmic origins. The study of phosphorus is not merely an academic pursuit but a reflection of the intricate connections that define our world, compelling us to delve deeper into the relationship between humanity and the elements that constitute our existence. Understanding the role of phosphorus enriches our appreciation for the natural world and the delicate balance of life, urging continued research and responsible practices to ensure its sustainability for generations to come.
